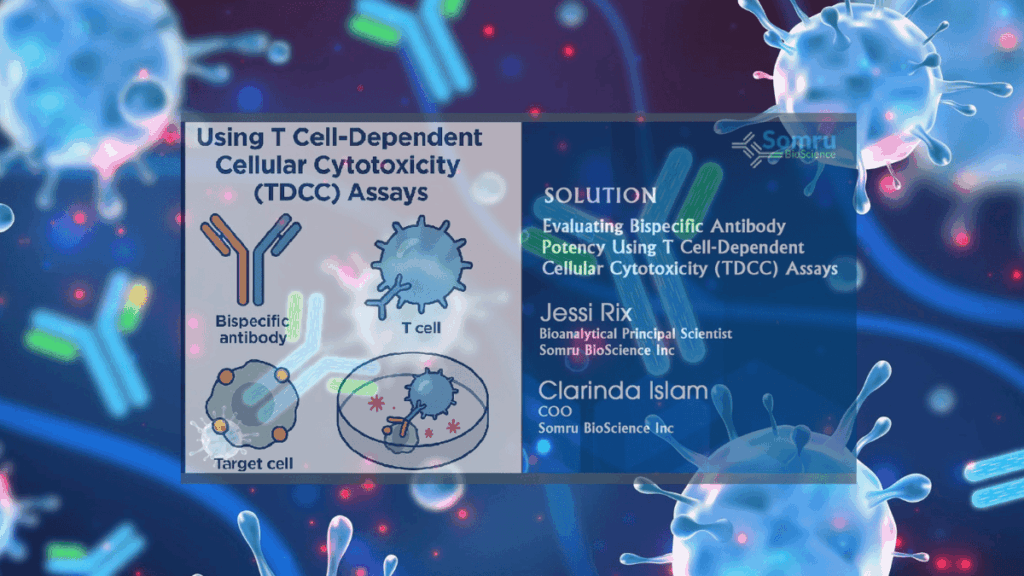
Discover how the TDCC (Target-Dependent Cell Cytotoxicity) assay offers a cutting-edge approach to evaluating antibody efficacy by accurately measuring immune-mediated tumor cell killing.
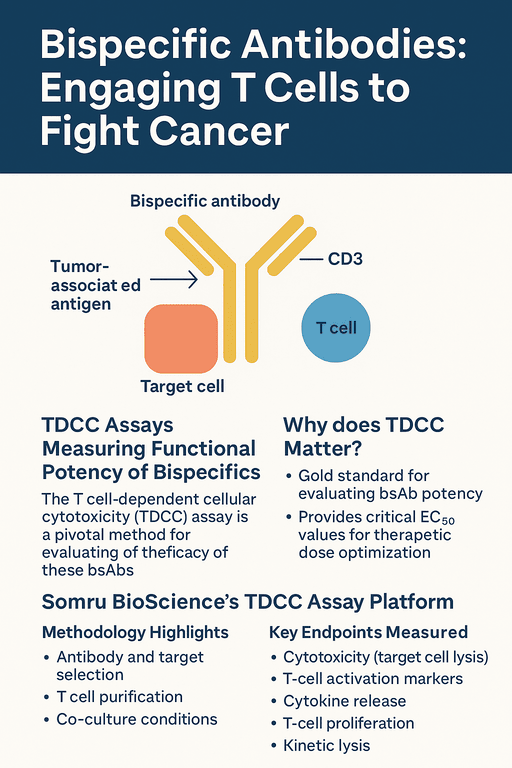
Bispecific antibodies (bsAbs) are an emerging class of cancer immunotherapies that can simultaneously bind a tumor-associated antigen and the CD3 receptor on T cells. This dual binding “engages” cytotoxic T cells with cancer cells, effectively connecting any T cell to a tumor cell regardless of the T cell’s native specificitypubmed.ncbi.nlm.nih.govpmc.ncbi.nlm.nih.gov. For example, blinatumomab – a CD19×CD3 bispecific for acute lymphoblastic leukemia – is already FDA-approved, illustrating the promise of this approachpmc.ncbi.nlm.nih.gov. In general, bsAbs can bypass normal MHC restriction and co-stimulatory requirements by driving T-cell activation and redirected tumor killing through their two binding armspmc.ncbi.nlm.nih.govpubmed.ncbi.nlm.nih.gov. By leveraging the body’s own immune effector cells, bsAbs have shown remarkable anti-tumor activity and occupy a growing role in precision oncology.
TDCC Assays: Measuring Functional Potency of Bispecifics
To develop and optimize bsAbs, it’s critical to test their functional activity in vitro. The T-cell–Dependent Cellular Cytotoxicity (TDCC) assay is the gold-standard cell-based assay for this purpose. In a TDCC assay, purified T cells and target tumor cells are co-cultured in the presence of the bsAb, and several functional readouts are measured. The assay directly quantifies the ability of the antibody to redirect T-cell–mediated killing of target cells. For example, Nazarian et al. describe a high-throughput TDCC assay that measures the percentage of target cell killing when a BiTE antibody engages T cells and redirects their cytolytic activitypubmed.ncbi.nlm.nih.gov. By testing a range of antibody concentrations, TDCC assays yield an EC_50 (half-maximal effective concentration) and dose–response profile, providing a precise potency metric for dose optimizationpubmed.ncbi.nlm.nih.goviqbiosciences.com.
In addition to direct cytotoxicity, TDCC assays can capture the molecular and kinetic hallmarks of T-cell engagement. Common secondary readouts include early activation markers (e.g. CD69, CD25, CD137) on CD4^+ and CD8^+ T cells, secretion of cytokines like IFN-γ, IL-2 and TNF-α, and proliferation of responder T cells. High levels of IFN-γ or IL-2 in the culture supernatant indicate strong immune activation, while cell-trace dyes (e.g. CFSE) can quantify T-cell proliferation. Advanced protocols even include real-time imaging or impedance-based platforms to monitor the kinetics of target cell lysis over timeiqbiosciences.compubmed.ncbi.nlm.nih.gov. Together, these endpoints give a comprehensive view of how effectively a bsAb recruits T cells and triggers an immune response.
Somru BioScience’s TDCC Assay Platform
At Somru BioScience, we have extensive experience designing TDCC assays to rigorously evaluate bispecific antibody candidates. Our approach leverages high-quality reagents and automation to maximize consistency and throughputsomru.ca. Target cells (cancer cell lines or engineered cells expressing the tumor antigen) are typically labeled and seeded in plates, then co‑cultured with purified pan-CD3^+ T cells isolated from healthy donor bloodiqbiosciences.com. Experiments use defined effector:target (E:T) ratios and incubation times tailored to the biology of the bsAb. We always include appropriate controls – for example, antigen-negative cell lines or isotype antibodies – to confirm that any observed killing is antigen-specific. Indeed, true T-cell engagement should only occur when both the bsAb and its cognate tumor antigen are present; for instance, no T-cell activation is seen when target cells lack the antigenpmc.ncbi.nlm.nih.gov.
By combining these elements, Somru ensures TDCC results that are robust and reproducible. We employ in-house automation solutions (robotic liquid handlers, automated incubators and readers) to improve consistency and accelerate timelinessomru.ca. Data are analyzed in real time, and dose–response curves are fitted to extract EC_50 and E_max values. Our scientists also monitor T-cell phenotypes by flow cytometry to detect upregulation of activation markers (e.g. CD69/CD25) and proliferation, and we use ELISA or MSD assays to quantify cytokines in the culture supernatantiqbiosciences.com. This multiparametric readout allows us to confirm the mode of action of each bispecific in development.
Methodology Highlights
- Antibody and target selection: We work with client-provided or in-house bsAbs and choose target cell lines with high and low antigen expression (or with engineered antigen variants). Targets are labeled (e.g. with cell trace dyes) to distinguish them from T cells during analysisiqbiosciences.com.
- T cell purification: Effector cells are purified human T cells (either pan-T or CD8^+ subsets) obtained from healthy donors or CRO sources. Purified T-cell populations reduce background variability compared to mixed PBMCs.
- Co-culture conditions: Target cells are plated first, and fresh T cells and serial dilutions of the bsAb are added to achieve the desired E:T ratio. Co-cultures are incubated (typically 24–72 hours) before analysis. We use both plate-reader (e.g. luminescent viability dyes) and flow cytometry readouts depending on the assay.
- Specificity controls: In parallel wells, we include control conditions such as (i) target cells without antigen, (ii) T cells alone, and (iii) positive controls (e.g. known BiTE antibodies). This confirms that killing and activation only happen in the full bsAb + antigen contextpmc.ncbi.nlm.nih.goviqbiosciences.com.
Key Endpoints Measured
- Cytotoxicity (target cell lysis): We measure loss of viable target cells by cell viability assays (e.g. LDH release, ATP/LumiLuciferase) or flow cytometry. A dose–response curve of percent lysis versus antibody concentration yields the EC_50 potencypubmed.ncbi.nlm.nih.goviqbiosciences.com.
- T-cell activation markers: Flow cytometry for CD69, CD25, CD137, etc., indicates rapid T-cell activation. Somru quantifies the percentage of T cells upregulating these markers in response to the bsAb. For example, one study showed that >80% of CD4^+ T cells became CD69^+ within 48 h only when both the BiTE and target antigen were presentpmc.ncbi.nlm.nih.gov.
- Cytokine release: We collect supernatants and measure Th1 cytokines (IFN-γ, IL-2, TNF-α) by ELISA or MSD. Elevated cytokine levels correlate with strong T-cell engagement. Importantly, Somru’s assays can track cytokine kinetics to assess safety (e.g. reduced cytokine storm risk) as well as potency.
- T-cell proliferation: Using proliferation dyes (e.g. CFSE), we assess whether the bsAb induces T cells to enter cell division. Proliferation can be a marker of sustained immune activation.
- Kinetic lysis: Advanced platforms (e.g. IncuCyte imaging or impedance systems) allow continuous monitoring of target cell killing. We can extract parameters like time-to-50%-lysis or area-under-curve, which provide insight into the speed of action of the bsAbiqbiosciences.com.
Each endpoint provides a different angle on bsAb activity. Combined, they paint a complete picture of efficacy and mechanism. All Somru TDCC assays are run under Good Laboratory Practice (GLP) conditions with rigorous data reporting, giving clients confidence in the results.
Conclusion and Call to Action
T-cell–dependent cellular cytotoxicity assays are a powerful preclinical tool for bispecific antibody development. By directly measuring how well a bsAb enlists T cells to kill tumor cells, TDCC assays guide lead optimization and dosing strategies, bridging in vitro activity to in vivo efficacy. Somru BioScience’s specialized TDCC platform—featuring purified T cells, validated target cell lines, and integrated endpoint analysis—enables biotech and pharma partners to accelerate their immuno-oncology programs.
To learn more about our capabilities, visit Somru’s CRO services page at somru.ca/cro and consult with a scientist todaysomru.ca. Our team stands ready to design a bespoke TDCC assay or other cell-based assay to meet your project goals, ensuring robust, reproducible data to advance your bispecific antibody from bench to clinic.