In bioanalytical laboratories, method validation is a time-intensive process that requires both scientific precision and regulatory rigor. To address these challenges, Somru BioScience Inc. has developed Aegyris—an innovative, web-based software solution tailored for comprehensive validation of pharmacokinetic (PK), anti-drug antibody (ADA), biomarker, and biosimilar assays. Aegyris integrates robust statistical analysis tools, real-time data visualization, and seamless third-party system compatibility into one streamlined platform.
Introduction
As the demand for biologics and biosimilars increases, the complexity of bioanalytical assays and the need for efficient method validation grow in tandem. Traditional approaches often rely on disparate tools, manual calculations, and isolated data workflows, leading to inefficiencies and inconsistencies. Aegyris aims to resolve these limitations through a unified, automated platform that supports scientists in conducting statistically sound, regulatory-compliant validations.
System Architecture and Design
Aegyris is built as a web-based application with a user-centric design. The frontend delivers a rich, interactive user experience for managing studies and analyzing data. The backend is powered by a scalable R statistical engine, capable of running on-demand analyses that comply with global regulatory requirements. Data storage is managed using a NoSQL database, which allows horizontal scalability and ensures performance under heavy computational loads.
A notable feature of Aegyris is its open interface module, which supports seamless data import/export from laboratory instruments and LIMS platforms. This interoperability ensures that users can maintain data continuity without extensive reformatting or manual entry.
Key Features
Aegyris offers a suite of analytical tools tailored to the specific needs of method validation in bioanalytical workflows:
- Normality Testing: Implements Shapiro-Wilk tests to evaluate data distribution.
- Log Transformation and Outlier Detection: Enables preprocessing and statistical integrity.
- Variance Analysis: Assesses consistency across replicates or conditions.
- Cut Point Determination: Offers mean and median-based approaches to determine thresholds, particularly critical in ADA and biomarker analysis.
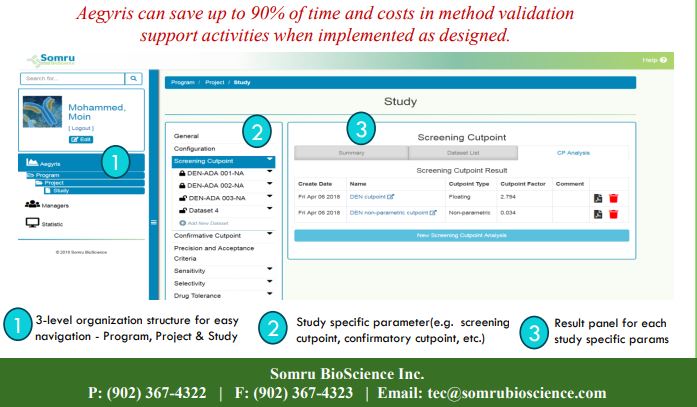
- Structured Navigation: A 3-tiered hierarchy—Program > Project > Study—supports scalable organization and traceability of results.
Each validation study is encapsulated in a well-defined unit, complete with parameter customization (e.g., screening and confirmatory cut points) and result panels for interpretation and reporting.
Performance and Efficiency Gains
By automating statistical workflows and integrating validation-specific functionality, Aegyris has demonstrated the potential to reduce time and cost associated with method validation by up to 90%. These savings stem from reduced manual effort, minimized transcription errors, and faster turnaround of results.
Conclusion
Aegyris represents a significant advancement in the field of bioanalytical method validation. Its integrated design, statistical rigor, and seamless interoperability make it an indispensable tool for laboratories handling PK, ADA, biomarker, and biosimilar studies. As the landscape of bioanalysis continues to evolve, platforms like Aegyris will be essential for maintaining both scientific excellence and regulatory compliance.
Contact:
Somru BioScience Inc.
📞 (902) 367-4322
📧 tec@somrubioscience.com


